D19
Instrument no longer available - Thermal neutron diffractometer for single-crystal and fibre diffraction
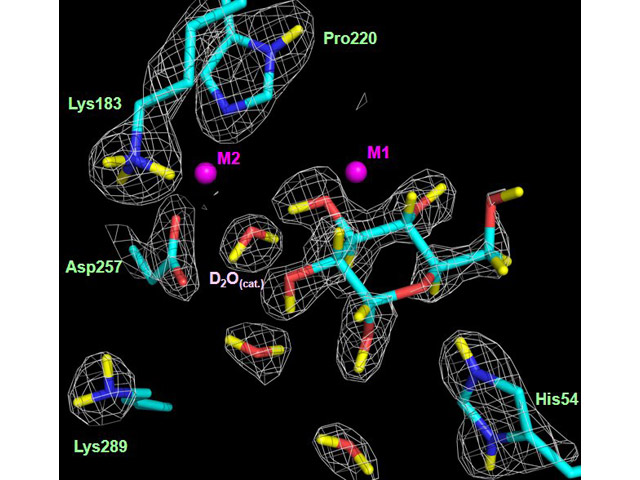
Neutron scattering density of the active-site residues and water molecules involved in the interactions between the enzyme xylose isomerase and glucose
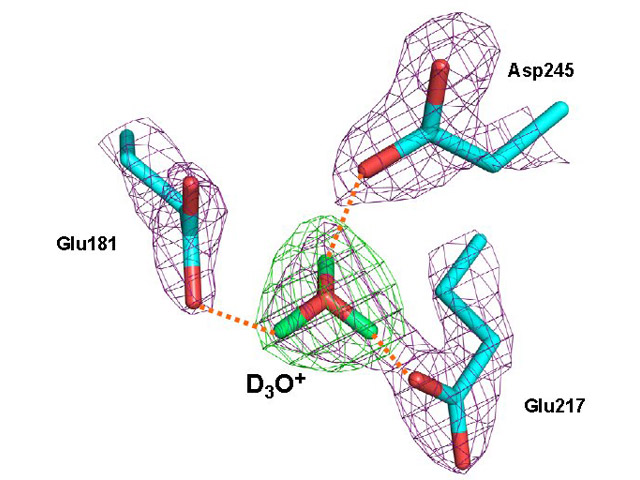
A close-up view of the putative hydronium (D3O+) cationThis is in XI-apo hydrogen bonded to three active site residues Glu181, Glu217 and Asp245 of xylose isomerase. The 2Fo-Fcneutron scattering d
Hydrogen transfer processes in the enzyme d-xylose isomerase
D-xylose isomerase (XI) catalyses the conversion of aldo to keto sugars. This occurs through a multi-step reaction involving the transfer of hydrogen. Structural information derived from X-ray crystallography has resulted in the proposal of several models for the reaction. These models differ in the way hydrogen is located and transferred.
The D19 diffractometer at the ILL has been used to carry out a detailed neutron crystallographic study with data extending to resolution of 1.4 Å.
This study provides direct information on the side-chain and ligand ionization states, and on the coordination of metal ions in complexes of XI that are important for the reaction mechanism. During ring opening, hydrogen is moved from O1 and to Lys289. During subsequent isomerization hydrogen is moved from the catalytic water, C2, and O2, and to O1 and C1, as a metal ion moves towards the aldehyde terminus of the linear sugar to bind to O1 and O2. These results have led to new suggestions as to how changes might take place over the course of the reaction (see Figure).
This work is the first study of its type using the monochromatic D19 diffractometer, and illustrates the scientific scope available when detailed information on hydrogen atoms and hydration is available in structural studies.
Ref.: A.Y. Kovalevsky, L. Hanson, S. Z. Fisher, M. Mustyakimov, S. A. Mason, V.T. Forsyth, M.P. Blakeley, D. Keen, T. Wagner, H.L. Carrell, A.K. Katz, J.P. Glusker, P. Langan, Structure – submitted & revised.
