Vast deposits of natural gas exist in the form of methane hydrate (MH), formed when water and methane molecules interact under specific pressure and temperature conditions. “The structure of methane hydrate – a solid compound similar to ice – allows large amounts of methane gas to be stored, caged within the lattice structure of water,” explains Mirian Casco, associate professor at the Universidad Católica del Uruguay. “I work on the development of energy storage systems inspired by this structure and designed to store methane, carbon dioxide or hydrogen.” The knowledge generated also guides research exploring the sustainable, environmentally safe and economically viable extraction of this untapped energy source that is located in frozen soils and deep-sea sediments.
With consolidated experience from a PhD at the University of Alicante (Spain) and a postdoctoral research position at the Dresden University of Technology (Germany), Casco integrated neutron techniques at the Institut Laue-Langevin (ILL) into her MH research after an introduction by Fernando Rey, professor at the Institute of Chemical Technology (ITQ) in Valencia, Spain. “Neutrons are particularly sensitive to hydrogen atoms,” explains Mónica Jiménez-Ruiz, ILL scientist at the responsible for the neutron vibrational spectrometer IN1-Lagrange. “The inelastic neutron scattering spectra measured using IN1-Lagrange provides information about the strength of the hydrogen bond network formed by water molecules, which is very different, for example, for confined or bulk water.”
For the MH energy storage system, inelastic neutron scattering provides insights into how the hydrogen atoms in the system behave within both the encapsulated methane gas molecules (CH4) and water molecules (H2O) of the lattice structure. “IN1-Lagrange enabled the vibrational bands of the entire system to be studied,” explains Jiménez-Ruiz. “The rotational bands of methane appear at low energies and could have been measured using other instruments at the ILL, such as IN5. IN1-Lagrange, however, is the only spectrometer at the ILL installed on the hot source and thus with access to the very high energy neutrons needed to measure the librational bands that are very sensitive to the hydrogen bond network and the intramolecular vibrations of the water molecules.”
Though conventional spectroscopic techniques, such as Raman or infrared, are capable of accessing the same energy range (0 to 500 meV), the water librational bands are generally masked by the signal from the porous framework. “The crucial contribution of neutrons is their unique ability to measure all vibrational bands, including librational, thus providing information that’s inaccessible using any other technique,” explains Jiménez-Ruiz.
Preliminary measurements to demonstrate feasibility were carried out and the proposal submitted by Casco and Jiménez-Ruiz was accepted by the ILL. “The kinetics of methane hydrate formation represents a significant challenge, it can take days or longer using bulk water,” explains Casco. The pores of adsorbent materials have been demonstrated to act as ‘nanoreactors’, accelerating MH formation. Two porous model carbons were thus employed: ordered mesoporous carbon (OMC) and hydrophilic ordered mesoporous carbon (HOMC). Both samples had similar pore structure (volume and shape) but differing surface chemistry: water-repelling hydrophobic (OMC) or water-attracting hydrophilic (HOMC).
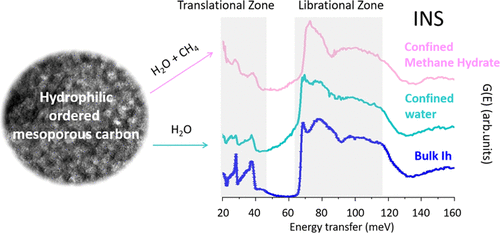
The carbon materials were first humidified such that the pores were either unsaturated (10-80% of the pore volume loaded with water) or oversaturated (150% of the pores filled) and then exposed to methane gas at 20 bar pressure and 200K (-73.15oC) for 1 hour. “Inelastic neutron scattering enabled the hydrogen atoms of water and methane hydrate confined in the pores of OMC and HOMC to be tracked,” explains Jiménez-Ruiz. “The vibrational features measured allowed the nature, structure and characteristics of confined water and methane hydrate to be identified in each environment: hydrophobic or hydrophilic.”
Hydrophilic materials result in more uniform surface coverage and INS spectra confirmed that water confined within the pores of unsaturated and oversaturated HOMC samples freezes into ice rich in stacking faults. These defects serve as active sites accelerating the formation of MH within the 1-hour timeframe studied. Conversely, nonfreezable water identified at the carbon pore wall of all HOMC samples and water outside the pores of oversaturated HOMC samples that behaves similarly to bulk ice, do not convert to MH within the 1-hour period. The hydrophobic surface of the OMC samples, on the other hand, induces the formation of large ice clusters with a structure similar to bulk ice and a lower level of nonfreezable water at the pore wall, neither of which convert to MH within the studied timeframe. Despite a similar level of stacking faults in the confined ice, the water-to-hydrate conversion remains incomplete after 1 hour.
“It was a challenging experiment to carry out, involving temperature and pressure control, as well as a gas sorption system,” explains Jiménez-Ruiz. “Furthermore, in addition to studying the vibrational bands of water, we were also looking to identify if, when and at which conditions methane hydrate formed within the system,” explains Casco. The results demonstrate that the yield of confined MH is significantly impacted by the surface chemistry of the porous model carbons, with formation accelerated by the hydrophilic environment.
The collaborative research continues in order to advance these energy storage systems using inelastic neutron scattering: a similar study has been carried out, with results due to be published soon, investigating mesoporous silica – a completely different material where the degree of hydrophobicity-hydrophilicity is more easily tuneable. Further experiments are also planned next year on IN1-Lagrange at the ILL to advance a methane/CO2 energy storage system using a biomass-based carbon platform. “The advantage of biomass materials is that they’re cheaper and can be more easily scaled up than the carbon or silica alternatives,” explains Casco.
ILL Instrument:IN1-Lagrange
Reference: Mirian E. Casco, Sven Grätz, En Zhang, Mónica Jiménez-Ruiz, and Lars Borchardt, The Journal of Physical Chemistry C 2024128 (25), 10281-10289
DOI: https://doi.org/10.1021/acs.jpcc.4c01082
ILL Contact: Monica Jimenez-Ruiz

