FIGARO
Fluid Interfaces Grazing Angles ReflectOmeter
Example 3: Solvent Extraction at Liquid/Liquid Interfaces
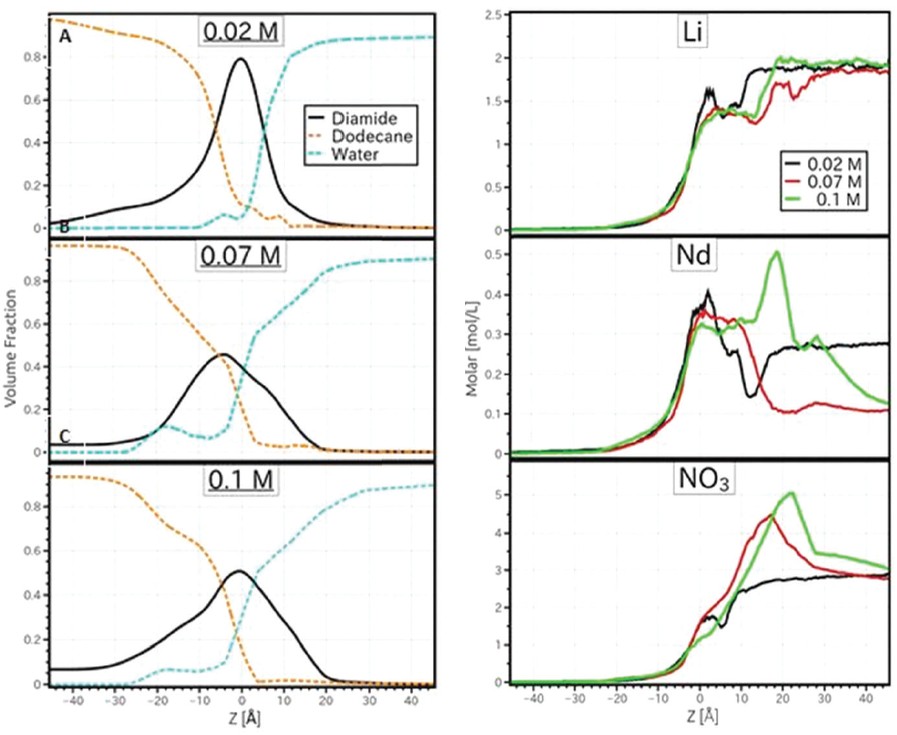
Interfaces between immiscible liquids are ubiquitous in chemistry and biology, and are the site of numerous physico-chemical processes such as sorption, solvation and complexation. Electron, electrolyte, molecule, or colloid transfer through liquid interfaces are relevant for domains like chemistry, nanoparticle synthesis and heterogeneous catalysis. In solvent extraction using amphiphilic ligands to separate ions, the ion partitioning between immiscible phases is enhanced by the ligand/ion complex formation on one side of the interface.
The combined application of neutron and X-ray reflectivity measurements represented a key milestone in the determination of the interfacial structure from two different lipophilic ligands. This combination is very helpful for understanding the microscopic structure at the LL interface. X-rays reveal the distribution of higher electron density aqueous solutes (salts, nitric acid), and neutrons are the perfect probes to quantify the structuration of protonated solvents and ligand molecules thanks to the use of isotopic substitution.
It was shown that hard trivalent cations can be repelled or attracted by the extractant-enriched interface according to the nature of the ligand. An interesting evolution of the interface with significant mixing of oil in water due to the presence of the extractant was also shown, which is a model that seems to be at odds with the current scheme of an amphiphilic monolayer.
The work was the first to report neutron measurements at the interface of two bulk liquids. It was made possible not only by the high flux of FIGARO to transmit through bulk liquid, but also by its ability to reach the interface from beneath, which enabled the crossing of heavy water below the interface and for hydrogenous ligands to be used in the oil phase, which would have resulted in too much attenuation for a configuration of neutrons reflecting upwards.
The results should serve as a basis for further understanding the extraction mechanism in order to improve the efficiency and kinetics of existing processes and to develop new ones. These methodological developments also allow the study of other liquid interfaces.