Neutron scattering techniques reveal the roles of SARS-CoV-2 fusion peptides during infection
Research has demonstrated the likely critical membrane fusion mechanism involved when the SARS-CoV-2 coronavirus infects human cells.
SARS-CoV-2, the virus responsible for the COVID-19 pandemic, is from a family of single-stranded positive sense RNA viruses named β-coronaviruses. SARS-CoV-2 and other β-coronaviruses can cause severe respiratory disease and are highly contagious. Despite this, a critical understanding of the mechanisms of cellular infection by coronaviruses has been lacking.
Researchers from the ILL, the University of Cambridge, the Italian National Research Council, and the Australian National Deuteration Facility have recreated important elements of the critical membrane fusion mechanism of the SARS-CoV-2 coronavirus by simplifying the system down to its core elements, amenable to experimental analysis by neutron scattering. Neutrons are well suited for the study of protein – membrane interactions under physiological conditions since they allow structural and dynamic characterization at room temperature. The research focused on the viral Spike protein, which plays an important role in infectivity. It has a strongly conserved membrane fusion domain, but the exact “fusion peptides” (short protein sequence involved in the fusion process) has not previously been conclusively identified.
The Spike protein can mediate cell entry either via direct fusion at the plasma membrane where calcium levels are high (~2mM), or at the endosomal membrane, where calcium levels are lower (~0.3μM). The ILL experiments on Spike fusion peptides provided direct structural information from specular neutron reflectometry and small angle neutron scattering, along with dynamic information from quasi-elastic and spin-echo neutron spectroscopy, to determine the molecular mechanisms of infectivity. Membranes were modelled using natural lipid extracts produced in a recently set-up facility within the Partnership for Soft Condensed Matter (PSCM) at the ILL. Many essential complementary measurements were also performed at the PSCM.
“Our multi-method approach reveals that different segments of the SARS-CoV-2 Spike protein assume different functions in the initiation of viral infection” claimed the team.
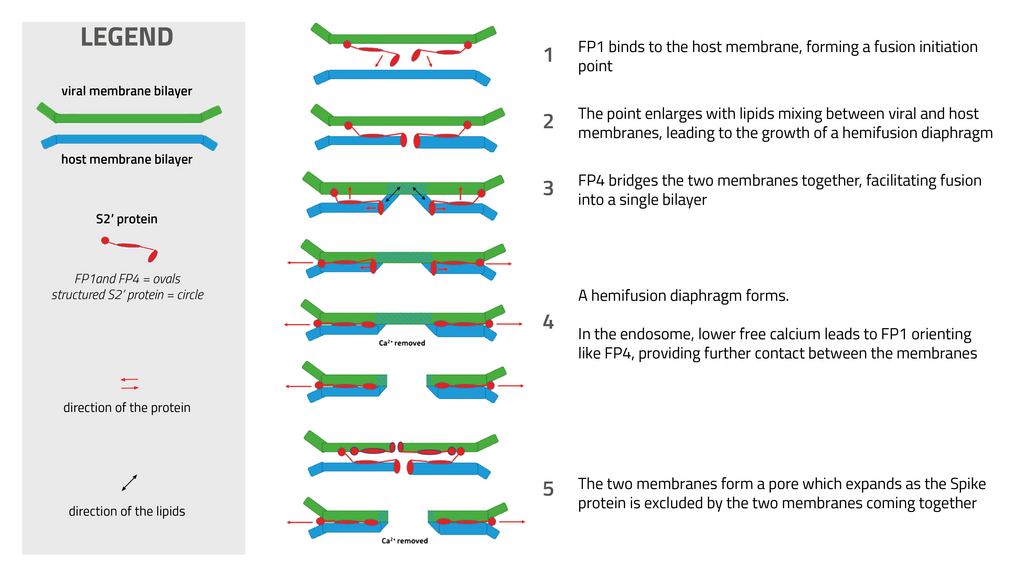
Proposed fusion mechanism between SARS-CoV-2 and eukaryotic host membrane.
The ILL data revealed peptides present within the fusion domain were found to interact primarily with lipid headgroups. The peptides act a bridge between the host and viral membranes and promote membrane fusion. However, in the presence of calcium, the N-terminal of the spike fusion domain harpoons through the lipid bilayer. It disorders the membrane on a picosecond time and Ångstrom length scales, forming a fusion initiation point where viral and host membrane lipids would start to mix. Intriguingly, on removing the calcium, the N-terminal fusion peptide orientation changes to a shallower position in the membrane, where it functions much more like the other fusion peptides studied, acting as a bridge between the host and viral membrane. The intracellular calcium levels may therefore provide an indication to where and how the viral and host membranes fuse during SARS-CoV-2 infection.
“Our data are of interest not only in the context of the current Covid-19 pandemic, but also provide a powerful interdisciplinary framework for future investigations of eukaryotic and viral fusion mechanisms” the research team highlighted.
Re.: “Strikingly Different Roles of SARS-CoV-2 Fusion Peptides Uncovered by Neutron Scattering", by Andreas Santamaria et al. ,J. Am. Chem. Soc. (2022).
The article can be accessed at https://doi.org/10.1021/jacs.1c09856
ILL instruments : Neutron reflectometer FIGARO, SANS instrument D22, Thermal neutron backscattering spectrometer IN13, Spin-echo spectrometer IN15, Backscattering spectrometer IN16B, cold neutron multichopper spectrometer IN5
Contacts: Andreas Santamaria, Armando Maestro, Daniela Russo, Giovanna Fragneto

 (jpg - 536 Ki)
(jpg - 536 Ki)