How batteries breathe
Neutron imaging provides real-time insights into the next generation of battery technology
The development of technological devices such as electric cars and smart devices happens before our very eyes at an almost breathtaking speed. The resulting quest for light, portable and high-performing batteries continuously drives battery innovation. Those based on the chemical elements lithium and sulfur (Li-S) are amongst the most promising next-generation candidates, mainly thanks to their ability to undergo rapid charge/discharge cycles.
The main components of a battery are two electrodes (known as cathode and anode) as well as a solution of charged particles, so-called electrolytes. This solution shuffles electrons between the two electrodes, driving the battery’s charge/discharge cycles. In the case of Li-S (lithium-sulfur) batteries, lithium particles are the ones responsible for providing energy. A low Li content (known as “lean electrolyte conditions”) is important for optimising the energy density of the battery, i.e. increasing the energy it can store within a given volume. Unfortunately, operating batteries under lean electrolyte conditions also makes the electrolyte solution dry out faster. This, in turn, often leads to a shorter battery lifetime.
Another crucial factor influencing Li-S battery performance is the coverage, also known as “wetting”, of the battery interior by the electrolyte solution. Wetting is so important since the electrochemical reactions driving battery performance mostly occur at the interface between the battery’s components and the solution. Poor wetting therefore increases the chance of cell failure.
To overcome these challenges and to optimise the design of future battery generations, detailed insights into the electrolyte distribution inside Li-S batteries are required. “Neutron imaging is the ideal technique for such investigations”, explains Yan Lu, the principal investigator of a study which was recently published in Advanced Energy Materials. “For this article, we looked at electrolyte wetting with Li-S cells while repeatedly charging and discharging the batteries.” These experiments, known as operando neutron imaging, were performed on ILL’s instrument NeXT.
Lu’s team was able to visualise the distribution of the electrolyte solution across different layers of Li-S batteries. Their experimental results revealed that the solution was distributed unevenly, affecting the battery’s performance. Before starting charging/discharging cycles (“cycling”), several regions untouched by the solution were observed. Importantly, discharging/charging strongly improved overall wetting. “Notably, we observed that the wetting occurred periodically, almost as if the battery was breathing the solution in and out”, emphasises Lu.
“Neutron imaging is an especially well-suited probe for such studies due to the sensitivity of neutrons to elements such as lithium and hydrogen, which play crucial roles in batteries and are notoriously hard to analyse with other techniques”, explains Lukas Helfen, a responsible of NeXT. This study – the first operando investigationoflean electrolyte batteries - is an important illustration of the unique advantages of neutron imaging in applied research aiming at developing future technologies.
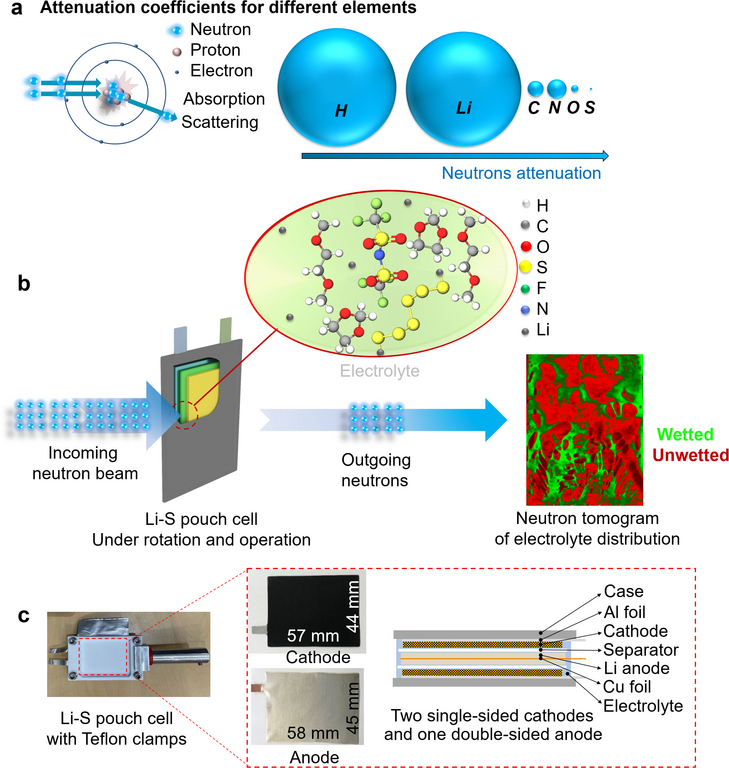
a) Schematic diagram of the interactions between neutrons and atoms, and the different attenuation coefficients for various main elements in the conventional ether-based Li-S electrolyte. The diameter of the spheres is proportional to the attenuation coefficients. b) Illustration of the imaging of the electrolyte when the neutron beam passing through a double-layer lithium-sulfur pouch cell. Green presents wetted regions; red, unwetted. c) The configuration of the studied pouch cell, containing two layers of single-sided cathode and one layer of double-side Li anode.
Reference: L. Lu, N. Kardjilov, X. Meng, K. Dong, Y. Xu, Q. Wu, A. Tengattini, L. Helfen, J. Yang, Y. Guo, M. Exner, I. Manke, Y. Lu, "Visualizing the Dynamic Wetting and Redistribution of Electrolyte in Lean-Electrolyte Lithium-Sulfur Pouch Cells via Operando Neutron Imaging", Advanced Energy Material (2025): e01324.
https://doi.org/10.1002/aenm.202501324
ILL instrument: NeXT
ILL contact persons: Alessandro Tengattini, Lukas Helfen

