

Next: Tables of Form Factors
Up: Magnetic Form Factors
Previous: Definition of form factors
The following tables give the coefficients of an analytic approximation
to the
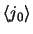 integrals for the d electrons in ions of the
3d
and 4d series, and the f electrons of some
rare earth and
actinide ions. The
approximation has the form
integrals for the d electrons in ions of the
3d
and 4d series, and the f electrons of some
rare earth and
actinide ions. The
approximation has the form
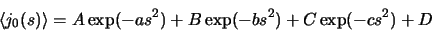 |
(7) |
For these expansions
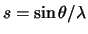 in units of Å-1.
in units of Å-1.
The integrals
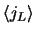 with
with 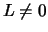 are zero when s=0 and have been
fitted with the form
are zero when s=0 and have been
fitted with the form
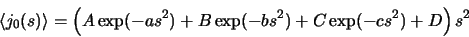 |
(8) |
The second set of tables give the coefficients obtained for
<j2> for 3d, and 4d transition metals,
htmlrefrare earthsrej2 and actinides,
<j4> for htmlref3d3dj4, and 4d transition metals,
rare earths and actinides,
and <j6> for rare earths and actinides.
For the transition metal series the fits were made with
form factor integrals calculated from Hartree-Fock wave-functions [2].
For
the rare-earth and actinide series the fits were with
Dirac-Fock form factors [3,4]. In the tables the number
following the atom symbol indicates the ionisation state of the atom.
Thus the coefficients following Fe0 are for a neutral iron atom and
those following Fe2 are for Fe2+.


Next: Tables of Form Factors
Up: Magnetic Form Factors
Previous: Definition of form factors
P.J. Brown - Institut Laue Langevin, Grenoble, FRANCE. e-mail: brown@ill.fr