Page 37 - ILL Annual Report 2019
P. 37
SCIENTIFIC HIGHLIGHTS
34-35
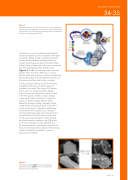
Figure 2
Thermal decomposition of multi-metal MOFs allow multi-cation oxides with compositions unattainable via conventional oxide synthetic routes to be obtained. The metal ratios in the resulting oxides are transferred from their corresponding parent MOFs.
Furthermore, we have since demonstrated that these complex compositions can be translated to other types of materials. Indeed, through a standard calcination process we have obtained multi-metal oxides that maintain the starting metal ratios of the parent MOFs. Notably, some of these metal oxides have compositions that are not attainable by other synthetic routes (figures 2 and 3). By combining single crystal and powder, X-rays and neutron diffraction, as well as electron microscopy techniques, we have completed the structural description of the starting MOF materials and fully characterised their metal oxides counterpart.
A strong correlation between the MOF initial metal arrangement and the type of oxide obtained is evidenced in this study. Thus, binary MOF systems tend to result in a mixture of crystalline phases, while for ternary ones the relative amount of cobalt in the MOF governs whether a mixture of phase
or pure spinel oxides is obtained. For quaternary systems, a complex interplay between all four elements is observed, making it possible to obtain
a large number of pure-phase spinel oxides with a variety of compositions. Through this methodology we have been able to produce up to seventeen multi-metal spinel-type oxides with compositions that are translated from the MOF precursor and which can be used as electrocatalysts for ORR. Although the relation between composition and ORR activity
is yet to be understood, we have identified up to
five solids that exhibit current density values that rival those of commercial platinum catalysts while showing excellent recyclability and stability in respect of poisoning with methanol.
Figure 3
SEM images of MOFs (left), and the corresponding calcination products (right), which are composed of the metal-oxides’ nanoparticles.
www.ill.eu