Investigating the secrets of greener hydrogen fuel production
Hydrogen fuel is seen as the zero-carbon fuel of the future. It has the potential to be stored easily and when it’s consumed in a fuel cell, such as in a car engine, it produces only water and heat as a waste product.
“Most hydrogen fuel is produced by splitting steam into hydrogen using natural gas, which produces large quantities of carbon dioxide. However, if cleaner methods to produce hydrogen become technically efficient and economically viable, hydrogen fuel can help contribute to a robust carbon-free future,” says Dr. Mohamed Zbiri, from ILL.
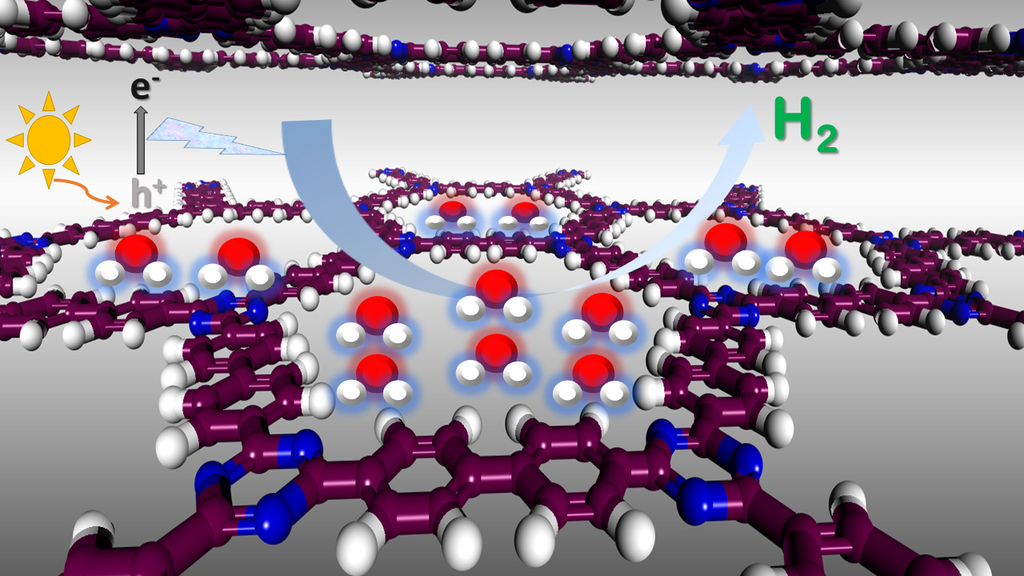
Hydrogen fuel generation can be achieved via the water-splitting process.A cleaner way of splitting hydrogen from water uses the energy from sunlight, rather than fossil fuels. This method uses photocatalysts to speed up the reaction, with the most common being inorganic semiconductors. However, scientists are now also investigating organic porous polymer photocatalysts. These are relatively easy to synthesise and process, allowing for precise control of their chemical design and therefore their functionality.“Chemistry by design is of great importance for the synthesis of targeted photocatalysts. The synthesis side of the project is done in collaboration with Prof. Andrew Cooper’s group at the University of Liverpool”, says co-researcher Dr. Anne Guilbert, from Imperial College London.
Organic porous photocatalytic materials resemble some naturally-occurring minerals like zeolites, in terms of their open structure sometimes called ‘molecular sieves’. “Organic porous water-splitting materials can be seen as ‘nanoreactors’,” explains Dr. Zbiri. “By accommodating water within their pores, ideally fuelled by solar energy, they trigger the process of splitting water molecules via oxidation and reduction reactions to release hydrogen.” The team studied two types of organic photocatalytic materials: covalent triazine-based frameworks (CTFs) and conjugated microporous polymers (CMPs).
The results were reported in two separate publications, which appeared recently in Chemistry of Materials on CTFsand inACS Applied Polymer Materials on CMPs.
Water-splitting depends strongly on the size and distribution of pores in these materials. More porous materials can provide a larger surface area for the water splitting, helping it happen in a fast and efficient way. “Our studies are focused on the link between the chemical design and the water mass transfer. The selection of the materials we studied is based mainly on their observed photocatalytic activities – i.e. how much water is taken up and how much hydrogen is subsequently produced - which are directly related to their structural aspects and behaviour subject to our neutron probe. Neutron spectroscopy was instrumental to the study,” says Dr. Guilbert.
The team used two neutron spectroscopy techniques - quasi-elastic neutron scattering (QENS) and inelastic neutron scattering (INS) - to look at how the water, which could be seen as ‘a guest’, moved through and interacted with the ‘host’ photocatalysts, on the picosecond time scale and nanometre space scale. “Neutrons are an excellent probe when probing organic materials made of light atoms such as hydrogen. Hydrogen exhibits the strongest signal here, so dynamics of water molecules could be clearly studied. The strong signal from hydrogen (H) forming the water can be tuned by using deuterium (D); an isotope of hydrogen, with a weaker neutron interaction than hydrogen to probe the response of the photocatalysts to the introduction of water. The use of D2O instead of H2O allows then to tune the contrast of the signal of the guest (water) and host (photocatalyst), as needed. QENS technique allowed us to examine the behaviour of the confined water, and the view is further strengthened by INS which allows observing how the water is interacting with the photocatalysts as well as to observe the induced response of the host materials to the presence of water. We used the IN5 spectrometer to study CTFs, and the IN6 and IN1-Lagrange spectrometers for the work on CMPs.,” says Dr. Zbiri
“Our microscopic probe of the local water environment and the related diffusion within pores revealed the state and behaviour of water. This is the first validation by experiment that the underlying atomistic mode of interaction of water is important”, says Dr. Guilbert.
The new studies have led to deeper and better insights into the behaviour of water and the response exhibited by the photocatalysts. The team hopes to take its work forwards by understanding further how the pore structures of materials affect their ability to split water into hydrogen fuel. Their ultimate aim is to help provide improved chemical design rules for creating better photocatalysts with enhanced water-splitting ability, thereby leading to more efficient clean hydrogen fuel generation.
Re1: Probing Dynamics of Water Mass Transfer in Organic Porous Photocatalyst Water-Splitting Materials by Neutron Spectroscopy.
Mohamed Zbiri et al. (2021). doi.org/10.1021/acs.chemmater.0c04425
Re2: Impact of Chemical Structure on the Dynamics of Mass Transfer of Water in Conjugated Microporous Polymers: A Neutron Spectroscopy Study.
Anne A. Y. Guilbert et al. (2021). doi.org/10.1021/acsapm.0c01070
ILL Instruments: Quasielastic Neutron Scattering (QENS) and Inelastic Neutron Scattering (INS) measurements were performed using the IN5 disk chopper time-of-flight spectrometer for the work on CTF photocatalysts, and using the IN6 time-focussing time-of-flight spectrometer and IN1-Lagrange for the work on CMP materials.
Contacts: Dr. Mohamed Zbiri, ILL – Dr. Anne Guilbert, Imperial College London