D1B
CRG - High intensity two-axis powder diffractometer
Neutron studies of materials for Ni-MH batteries
Batteries work by converting chemical energy stored in electrodes into electrical energy, via electrochemical reactions. Neutrons can easily penetrate the sample so as to give information about the bulk material, and the hydrogen and lithium ions trapped in the electrodes scatter neutrons so they can be readily located.
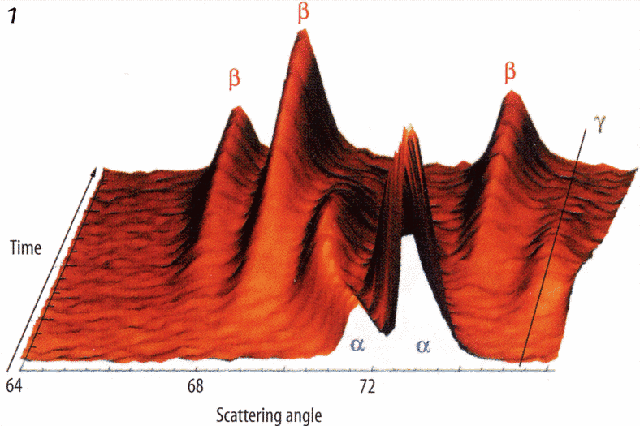
Extensive studies on nickel-lanthanum hydrides used as electrode materials in Ni-MH batteries have been carried out. The continual charge-discharge of the standard electrode involves a cyclic transformation between two crystalline phases called α and β. These have unit (cell) volumes that differ by 20 per cent, which induces heavy constraints in the material and causes its crystal structure to fragment.
That leads to surface corrosion and reduces the battery lifetime. Experiments made on D1B followed changes during cycling on different electrode materials. These experiments showed that a transitory intermediate γ phase with a cell volume between that of the α and β phases may appear (see figure 1) for some peculiar alloy compositions. The appearance of this intermediate γ phase at the boundary between the α and β phases significantly reduces the constraints during the cycling process, leading to a better lifetime.
Ref.: M. Latroche, Y. Chabre, A. Percheron-Gueguan, O. Isnard, B. Knosp, J. of Alloys and Comp. 330-332 (2002) 787-791.