D10
Four-circle diffractometer with three-axis energy analysis
The structure of a cage compound at 30K
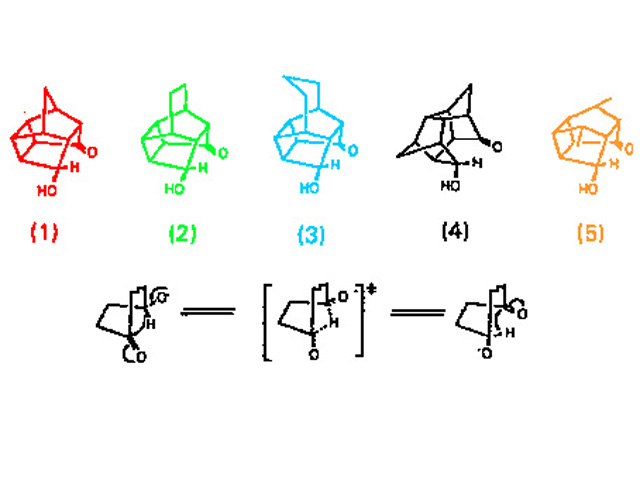
The use of crystallographic data to map reaction pathways is well established for interactions of nitrogen and oxygen nucleophiles with carbonyl, both in bond formation and cleavage. Could a similar analysis by structural correlation be applied to the reduction of ketonic carbonyl by hydride addition?
A number of polycylic hydroxyketones, containing sterically constrained 4-hydroxycyclohexanone or 4-hydroxycycloheptanone substructures which rearrange by a 1,4 hydride shift on treatment with base (Fig. 1), were prepared.
Rates of rearrangement of the derived alkoxides had been determined, as had the X-ray crystal structures of derivatives of some of the hydroxyketones. Crucial to the analysis of the correlations were the positions of the potentially hydridic hydrogens at the alcohol methine, and should ideally be determined by neutron diffraction. This experiment was the first such determination of the structure of a reactive hydroxyketone.
This analysis gave clear evidence of steric compression of the potential hydride donor and accepting carbonyl (Fig. 2).
Somewhat surprisingly there was no evidence of any adjustment in molecular geometry to show coupling between the functional groups, a result attributed to the weaker hydridic character of this alcohol compared to its salts.
Ref.: R.H. Fenn et al., Acta Cryst. C45 (1989) 423-428.

![2- The two independent dimers of the cage compound5-hydroxy-7,10-dimethyltetracyclo[4.4.0.03,9.04,8]decan-2-one (C12H16O2)These are viewed from similar directions nad o : denotes](/fileadmin/user_upload/ILL/3_Users/Instruments/Instruments_list/00_-_DIFFRACTION/D10/img/d10-example5.jpg)